The infectious agent in diseases such as scrapie and Creutzfeldt-Jacob disease contains no nucleic acid. How do it manage to replicate without any DNA or RNA? Information appears to be stored in the structure of the protein aggregate or prion. Prion aggregates can grow by incorporating new prion protein (PrP) and inducing it to refold into the pathological prion form. Growth of prion aggregates, however, is not enough for replication. At some point, one prion must become two prions.
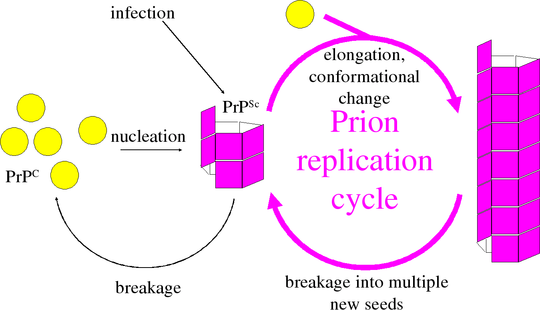
The kinetics of elongation and breakage are exponential over time, and quite different to the previously studied kinetics of nucleation and growth. Nucleation is a very rare process, and can generally be ignored in vivo, since disease usually begins with an infectious introduction. Even when disease is spontaneous, intervention will always be too late to interfere with nucleation. Instead, we focus on the exponential rate of growth.
Prion diseases have incubation periods as long as decades in humans. It would be enough to slow these down even a little, rather than find a complete “cure”. We developed a mathematical model that captures the combined kinetics of growth and breakage to calculate the exponential growth rate.
The incubation period of prion diseases in lab conditions is remarkably reproducible, to an extent that may be unprecedented in in vivo biology. Using this precise data, we tested our model’s predictions as to how the incubation period varies as a function of PrP gene dosage, inoculum dose, and the aggregation state of the inoculum. In all cases, our model has fit the data well, and we have been able to estimate all the parameters of the model. Our model of exponential replication, which predicts a square root dependence on the PrP concentration, was also found to be a good fit to in vitro data (Knowles et al. 2009).
We are also able to predict the effect of drugs of different classes on the incubation time. Drugs that cap the growing ends of prion aggregates are by far the most promising class of drugs, according to our results. Such drugs, which may work by mimicking the normal form of PrP, are the most likely to be effective (ie, lower the exponential growth rate and hence the incubation period) at low drug doses.
Publications:
- Masel, J., Genoud, N., & Aguzzi, A. (2005). Efficient inhibition of prion replication by PrP-Fc2 suggests that the prion is a PrPSc oligomer. J Mol Biol, 345(5), 1243-51. (PubMed)
 (doi)
(doi)
- Masel, J., & Jansen, V. A. (2004). Prion kinetics. Biophys J, 87(1), 728. (PubMed)
 (doi)
(doi)
- Masel, J., & Jansen, V. A. (2001). The measured level of prion infectivity varies in a predictable way according to the aggregation state of the infectious agent. Biochim Biophys Acta, 1535(2), 164-73. (PubMed)

- Masel, J., & Jansen, V. A. (2000). Designing drugs to stop the formation of prion aggregates and other amyloids. Biophys Chem, 88(1-3), 47-59. (PubMed)

- Masel, J., Jansen, V. A., & Nowak, M. A. (1999). Quantifying the kinetic parameters of prion replication. Biophys Chem, 77(2-3), 139-52. (PubMed)

- Masel, J., & Jansen, V. A. (1999). The kinetics of proteinase K digestion of linear prion polymers. Proc Biol Sci, 266(1431), 1927-31. (PubMed)
 (doi)
(doi)