|
D. Lawrence Venable Department of Ecology and Evolutionary Biology University of Arizona Tucson, AZ 85721 OFFICE (520) 621-5956; FAX (520) 621-9190 EMAIL venable@email.arizona.edu |
|
Home | Research | People | Publications | LTREB Data Sets | Links | Seed Images |
LTREB
Data Set
| ||||||
There are two archives accessible from this page. One is an archive of the data set constituting all information associated with the Spalding-Shreve plant-monitoring program at the U.A. Desert Laboratory in Tucson from 1906 through the updated 2012 census. This is the oldest individual-based plant monitoring program in the world (Rodriguez et al. 2013). Analyses derived from these data have been historically pivotal in testing theories on plant succession, life history traits, longevity, and population dynamics (see Shreve and Hinckley (1937), Murray (1959) and Bowers (2002 and 2005 a, b), Goldberg and Turner (1986), and Butterfield et al. (2010)). This archive was recently published as an Ecology Archives Data Paper and can be accessed at this link: One hundred and six years of population and community dynamics of Sonoran Desert Laboratory perennials. The second archive describes a long-term data set on the population dynamics of desert annuals collected by D. L. Venable at the Department of Ecology and Evolutionary Biology, University of Arizona. It can be accessed at this link: Desert Annual Archive. The data is presented in Ecology Archives Data Paper format with metadata following Michener et al. (1997). All data files are in .csv format. Below is a brief description of some of the research we have done in relation to this data set. Population and community
ecology of desert winter annuals - The main
goal of this research was to determine how desert annuals adapt to and
coexist in variable and unpredictable environments. The resulting long
term population and community dynamic data has formed the backbone of
a synthetic research program on desert annuals combining evolutionary,
physiological, population and community ecological approaches to answer
questions uniting functional biology, life history evolution and community
dynamics. Figure.
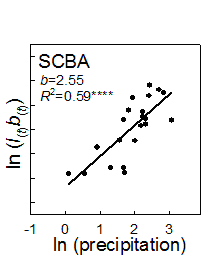
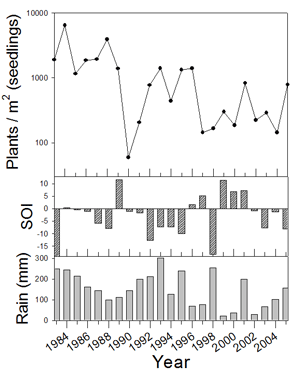
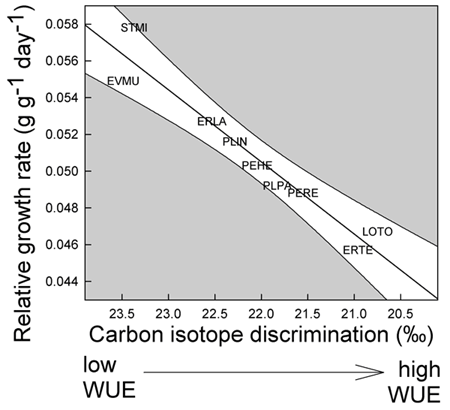
1. Example of weather linkage. However, exceptions exist and species have individualistic responses to temporal variation (Venable et al. 1993, Pake and Venable 1995, Venable and Pake 1999). The total number of seedlings of all species emerging has varied by more than two orders of magnitude over the 24-year period with a multidecadal decline evident (Fig. 2).
Figure 2. Density ofl
seedlings of all species (at emergence)
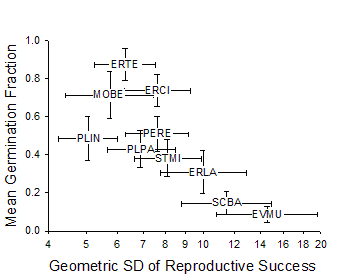
Figure 3. Mean germination
fraction vs. related to variation Adondakis, S., and D. L. Venable. 2004. Dormancy and germination in a guild of Sonoran Desert annuals. Ecology 85:2582-2590. Angert, A. L., T. E. Huxman, G. A. Barron-Gafford, K. L. Gerst, and D. L. Venable. 2007. Linking growth strategies to long-term population dynamics in desert annuals. Journal of Ecology 95:321-331. Angert, A.L., T. E. Huxman, P. Chesson, and D. L. Venable. In Review. Functional tradeoffs determine species coexistence via the storage effect. Nature. Barron-Gafford, G. A., A. L. Angert, D. L. Venable, A. P. Tyler, K. L. Gerst, and T. E. Huxman. in review. Photosynthetic temperature responses of desert annuals with contrasting resource-use efficiencies. Oecologia. Bowers, J. E. 2005. Effects of drought on shrub survival and longevity in the northern Sonoran Desert. Journal Of The Torrey Botanical Society 132:421-431. Bowers, J. E., and R. M. Turner. 2001. Dieback and episodic mortality of Cercidium microphyllum (foothill paloverde), a dominant Sonoran Desert tree. Journal Of The Torrey Botanical Society 128:128-140. Bowers, J. E., and R. M. Turner. 2002. The influence of climatic variability on local populations dynamics of Cercidium microphyllum (foothill paloverde). Oecologia 130:105-113. Bowers, J. E., R. M. Turner, and T. L. Burgess. 2004. Temporal and spatial patterns in emergence and early survival of perennial plants in the Sonoran Desert. Plant Ecology 172:107-119. Chesson, P. 1994. Multispecies Competition In Variable Environments. Theoretical Population Biology 45:227-276. Chesson, P. 2000a. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics 31:343-366. Chesson, P. 2000b. General theory of competitive coexistence in spatially-varying environments. Theoretical Population Biology 58:211-237. Chesson, P., and N. Huntly. 1989. Short-term instabilities and long-term community dynamics. Trends In Ecology & Evolution 4:293-29 Chesson, P. L., and N. Huntly. 1988. Community Consequences Of Life-History Traits In A Variable Environment. Annales Zoologici Fennici 25:5-16. Clauss, M. J. 1999. Life history strategies in variable environments. Ph.D. Dissertation. University of Arizona, Tucson. Clauss, M. J., and D. L. Venable. 2000. Seed germination in desert annuals: An empirical test of adaptive bet hedging. American Naturalist 155:168-186. Ellner, S. 1987. Alternate Plant Life-History Strategies And Coexistence In Randomly Varying Environments. Vegetatio 69:199-208. Ellner, S., and A. Shmida. 1981. Why are adaptations for long-range seed dispersal rare in desert plants. Oecologia 51:133-144. Evans, M. E. K., and J. J. Dennehy. 2005. Germ banking: Bet-hedging and varlable release from egg and seed dormancy. Quarterly Review Of Biology 80:431-451. Evans, M. E. K., R. Ferriere, M. J. Kane, and D. L. Venable. 2007. Bet hedging via seed banking in desert evening primroses (Oenothera, Onagraceae): Demographic evidence from natural populations. American Naturalist 169. Grubb, P. J. 1977. Maintenance Of Species-Richness In Plant Communities - Importance Of Regeneration Niche. Biological Reviews Of The Cambridge Philosophical Society 52:107-145. Holzapfel, C., and B. E. Mahall. 1999. Bidirectional facilitation and interference between shrubs and annuals in the Mojave Desert. Ecology 80:1747-1761. Hopper, K. R. 1999. Risk-spreading and bet-hedging in insect population biology. Annual Review Of Entomology 44:535-560. Hutchinson, G. E. 1961. The Paradox Of The Plankton. American Naturalist 95:137-145. Huxman, T. E., G. A. Barron-Gafford, K. L. Gerst, A. L. Angert, A. P. Tyler, and D. L. Venable. 2008. Photosynthetic resource-use efficiency and demographic variability in desert annuals. Ecology 89:In Press. Michener, W. K., J. W. Brunt, J. J. Helly, T. B. Kirchner, and S. G. Stafford. 1997. Nongeospatial metadata for the ecological sciences. Ecological Applications 7:330-342. Moriuchi, K. S., D. L. Venable, C. E. Pake, and T. Lange. 2000. Direct measurement of the seed bank age structure of a Sonoran Desert annual plant. Ecology 81:1133-1138. Mun, H. T., and W. G. Whitford. 1997. Changes in mass and chemistry of plant roots during long-term decomposition on a Chihuahuan Desert watershed. Biology And Fertility Of Soils 26:16-22. Pake, C. E., and D. L. Venable. 1995. Is Coexistence Of Sonoran Desert Annuals Mediated By Temporal Variability In Reproductive Success. Ecology 76:246-261. Pake, C. E., and D. L. Venable. 1996. Seed banks in desert annuals: Implications for persistence and coexistence in variable environments. Ecology 77:1427-1435. Pantastico, M. C. 1991. Competition in desert winter annuals: effects of spatial and temporal variation. Ph.D. Dissertation. University of Arizona, Tucson, AZ. Pantastico-Caldas, M., and D. L. Venable. 1993. Competition in two species of desert annuals along a topographic gradient. Ecology 74:2192-2203. Pierson, E. A., and R. M. Turner. 1998. An 85-year study of saguaro (Carnegiea gigantea) demography. Ecology 79:2676-2693. Rees, M., R. Condit, M. Crawley, S. Pacala, and D. Tilman. 2001. Long-term studies of vegetation dynamics. Science 293:650-655. Shmida, A., and S. Ellner. 1984. Coexistence Of Plant-Species With Similar Niches. Vegetatio 58:29-55. Suding, K. N., D. E. Goldberg, and K. M. Hartman. 2003. Relationships among species traits: Separating levels of response and identifying linkages to abundance. Ecology 84:1-16. Tilman, D., and S. Pacala. 1993. The maintenance of species richness in plant communities. in R. E. Ricklefs and D. Schulter, editors. Species diversity in ecological communities. University of Chicago Press, Chicago. Turnbull, L. A., M. Rees, and M. J. Crawley. 1999. Seed mass and the competition/colonization trade-off: a sowing experiment. Journal Of Ecology 87:899-912. Tyler, A. P., T. E. Huxman, and D. L. Venable. In Prep. Winter annuals effect fertile island development and perennial plant function in the Sonoran Desert. Venable, D. L. 2007. Bet hedging in a guild of desert annuals. Ecology 88:1086-1090. Venable, D. L., and J. S. Brown. 1988. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. American Naturalist 131:360-384. Venable, D. L., and J. S. Brown. 1993. The Population-Dynamic Functions Of Seed Dispersal. Vegetatio 108:31-55. Venable, D. L., A. Flores-Martinez, H. C. Muller-Landau, G. A. Barron-Gafford, and J. X. Becerra. 2008. Seed dispersal of desert annuals. Ecology. Venable, D. L., C. E. Pake, and A. C. Caprio. 1993. Diversity and coexistence of Sonoran desert winter annuals. Plant Species Biology 8:207-216. Venable, D. L., and C. E. Pake. 1999. Population ecology of Sonoran Desert annual plants. Pages 115-142 in R. H. Robichaux, editor. The ecology of Sonoran Desert plants and plant communities. University of Arizona Press, Tucson. Venable, D. L., C. E. Pake, and A. C. Caprio. 1993. Diversity and coexistence of Sonoran desert winter annuals. Plant Species Biology 8:207-216. Warner, R. R., and P. L. Chesson. 1985. Coexistence Mediated By Recruitment Fluctuations - A Field Guide To The Storage Effect. American Naturalist 125:769-787. |